Project Quality Control
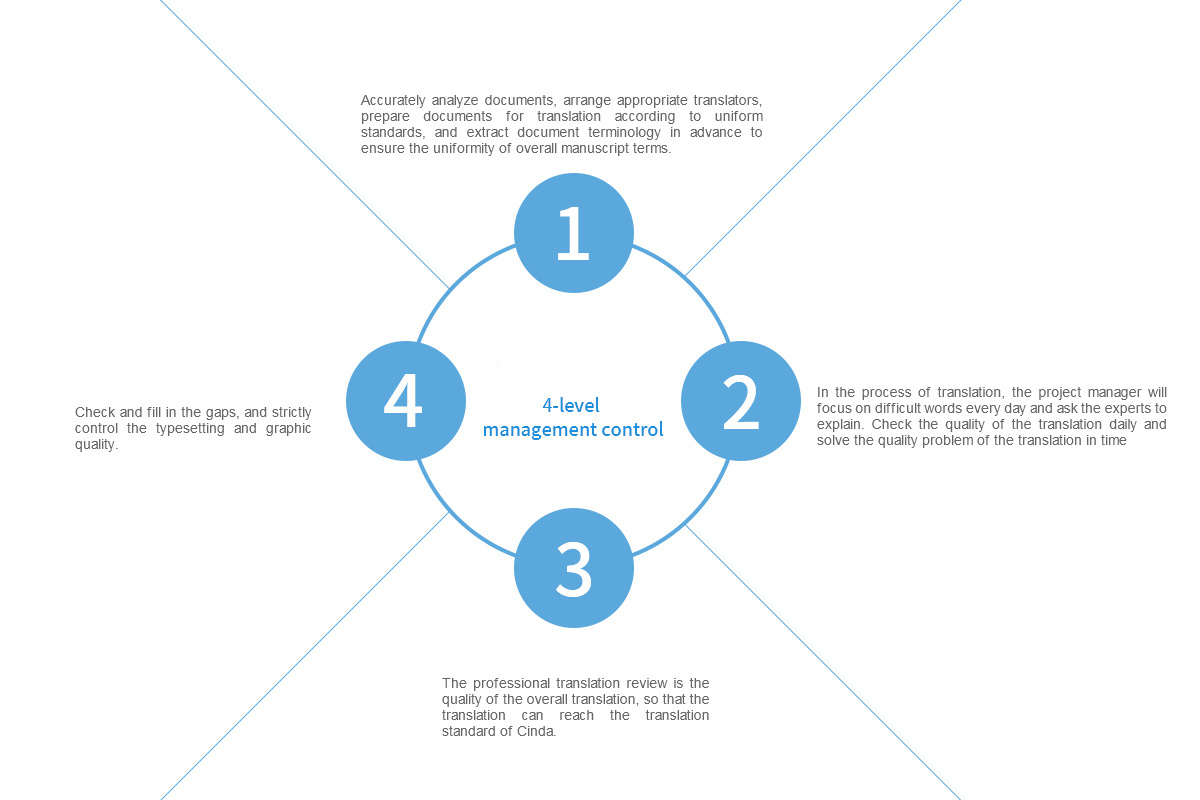
We have reached the GB/T19363.1-2022 international/domestic standards, passed the ISO9001 translation quality system certification and ISO17100 translation management system certification, and established project plans and project supervision management systems for all medical and pharmaceutical translation documents undertaken, ensuring the translation quality and time requirements of each project, strictly implementing a four-level sixteen-step quality management control and three-review audit system to guarantee the high-quality output of the translations undertaken.



Follow us
Hello, what can we do for you?
* Note:Today's message has a discount,Please be sure to fill in the information accurately and keep the communication unblocked. We will get in touch with you as soon as possible.
COOKIES
Our website uses cookies and similar technologies to personalize the advertising shown to you and to help you get the best experience on our website. For more information, see our Privacy & Cookie Policy
COOKIES
Our website uses cookies and similar technologies to personalize the advertising shown to you and to help you get the best experience on our website. For more information, see our Privacy & Cookie Policy
These cookies are necessary for basic functions such as payment. Standard cookies cannot be turned off and do not store any of your information.
These cookies collect information, such as how many people are using our site or which pages are popular, to help us improve the customer experience. Turning these cookies off will mean we can't collect information to improve your experience.
These cookies enable the website to provide enhanced functionality and personalization. They may be set by us or by third-party providers whose services we have added to our pages. If you do not allow these cookies, some or all of these services may not function properly.
These cookies help us understand what you are interested in so that we can show you relevant advertising on other websites. Turning these cookies off will mean we are unable to show you any personalized advertising.
